A Rapid And Cost-Effective Way To Gain Actionable Insights That Drive Clinical Trial Success

What Is The NetraAI Lab?
The NetraAI Lab is 4 week accelerated insight generator experience that will provide clinical development teams access to intelligence using their own historical data for a single clinical stage asset.
Key Benefits
- QUALITY: Enhance signal detection to reveal explainable patient subpopulations leading to study design recommendations to increase effect sizes and lower p-values
- ACCURACY: Improve accuracy of patient population heterogeneity and improve predictability. Accelerate the pace of innovation by leveraging AI to streamline your clinical development processes.
- SPEED: Fast-track your drug development with intelligent insights to enrich your protocol design leading to more effective patient matching strategies that can de- risk and accelerate late phase development.
- SAFETY: Identify patient factors underlying adverse events to improve patient monitoring and avoid safety issues that can delay programs
Deliverables
Sample variable characterization and explainable subpopulations
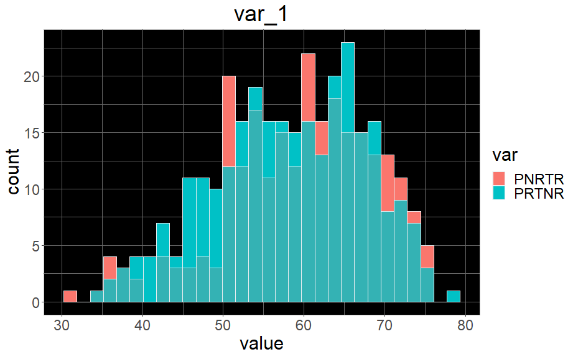
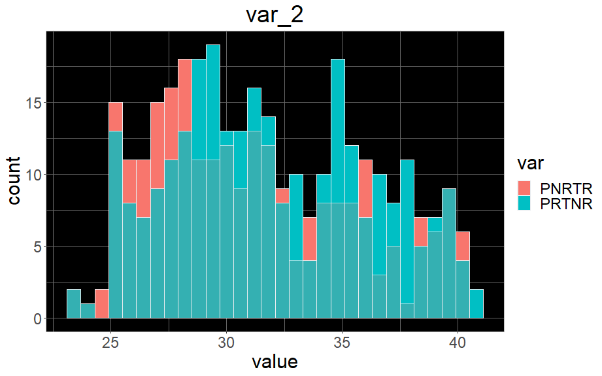
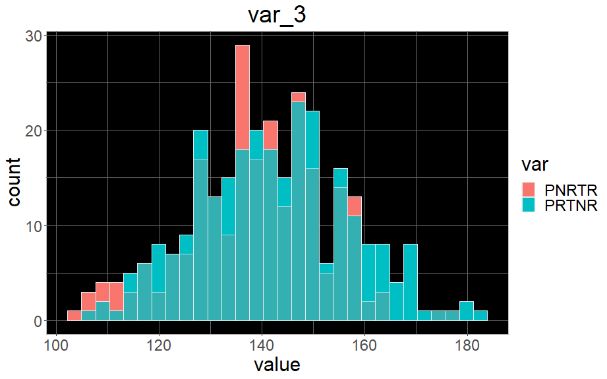
Sample selection criteria based on NetraAI analysis
Inclusion Criteria | Expected Effect on Study Population | |
DARS13 score > 1 (Appetite generally maintained) | • Removes many non responders | • Some increase in screen failure rate |
DARS14 score >1 (appetite generally maintained) | • Removes many non responders • Small effect drug responders | • Some increase in screen failure rate |
QLESQ7 >2 (appetite generally maintained) | • Removes many non responders • Smaller effect on drug responders | • Increases screen failure rate |
QLESQ2 >2 (exclude those very dissatisfied with mood) | • Removes many non responders • Small effect drug responders | • Some increase in screen failure rate |
MADRS: Reduced Appetite > 2 (appetite generally maintained) | • Removes many non responders • Small effect drug responders | • Some increase in screen failure rate |
SHAPS2 < 2 (pleasure in relationships maintained) | • Removes many non responders • Small effect drug responders | • Some increase in screen failure rate |
Supine Respiration > 16 | • Removes most placebo responders • Removes some drug non-responders | Increase in screen failure rate |
HGB < 160 | • Removes many placebo responders • Has only a mild effect on removing | Slight increase in screen failure rate |

“Our mission at NetraMark is to have a meaningful impact on the clinical trial process. Our singular focus has been on building advanced AI solutions that leverage clinical study data and affect the quality, accuracy, speed and safety of late stage trials. Our proven NetraAI has been leveraged to “see insights” that de-risk clinical trials by providing advanced personas that are important for various stages of clinical trial planning and execution that can accelerate drug development, and bring safer and more effective treatments to market”
-George Achilleos, CEO NetraMark
