Alzheimer’s disease (AD) is a neurodegenerative disease that contributes to 60-70% of all dementia cases and is characterized by neuronal cell damage and cognitive decline.1 Currently, drugs for AD target symptom control and there is no effective curative therapy for the disease. Given that prevalence of AD is expected to rise as the population ages, there is an urgent need to identify curative and restorative therapies.
One potential reason that AD therapeutics have failed to advance is due to gaps in knowledge of the complex genomic and environmental factors that contribute to AD. Herein lies an opportunity for a multimodal approach to account for the numerous factors contributing to AD initiation and progression and generate a precision medicine approach for the diagnosis, classification, and treatment of AD.
The Role of Machine Learning in Alzheimer’s Disease
Machine learning (ML) methods allow us to take a systems-level approach to analyzing multimodal data from the AD population. This approach accounts for complex genetic interactions and environmental factors to identify covariates and clusters of factors that provide as of yet undiscovered classification of disease and potential therapeutic targets. Most ML studies in AD have been focused on AD risk and prediction of progression of cognitive impairment using brain imaging and genomic data, with less work focused on classification of genetically determined subtypes within the AD patient population.
The NetraAI Advantage
NetraAI employs a suite of ML techniques that have the ability to learn from smaller datasets compared to conventional ML approaches. There are two primary novel components to NetraAI:
-
-
A data representation technique that is able to learn about the various ways that patients relate to each other without backpropagation.
-
A feature selection methodology that favors finding combinations of variables that act together to define subpopulations of interest.
-
In addition to this, we employ standard methods such as ensemble trees and kernel methods. This enables the extraction of valuable insights that accurately reflect the studied population.
The unique advantage of NetraAI is its capability to discover unknown subpopulations that are defined by multiple genes and provide explanations for underlying driving mechanisms behind each subpopulation.2 Furthermore, NetraAI can analyze a patient population from various angles, offering different perspectives for modeling groups of AD subjects based on different sets of genes. This approach is referred to as perspective analytics, and each perspective is learned by the machine and consists of a unique set of variables with varying contributions.
Using a publicly available AD dataset (GSE84422), consisting of transcriptomic data from 356 postmortem brain samples from different brain regions from AD subjects and healthy controls, we were able to extract deeper insights into AD subtypes beyond APOE, which has already been widely explored and well-established in the field.3 4
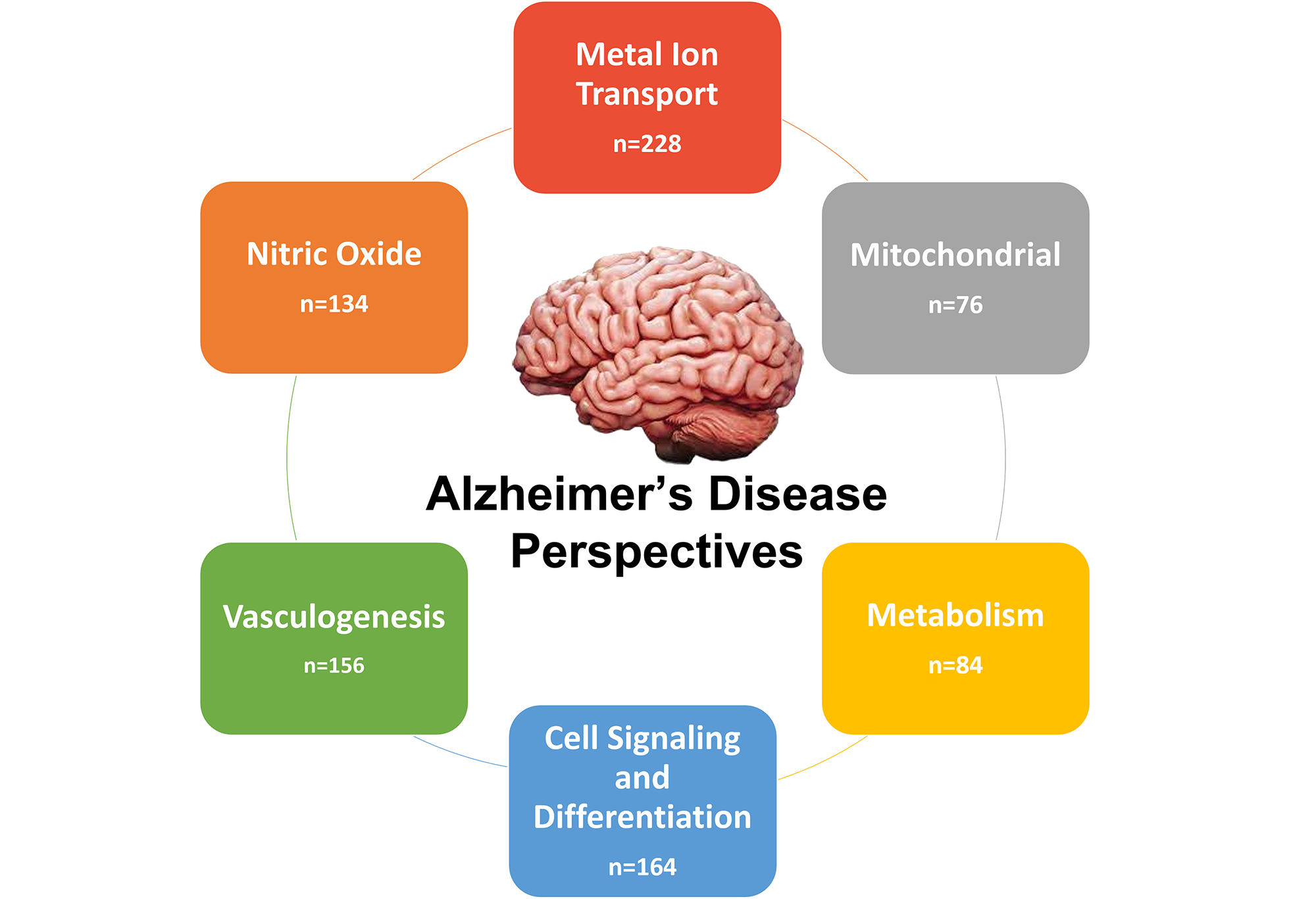
Figure 1. NetraAI-identified perspectives in Alzheimer’s Disease.
An individual can experience or progress to AD through a single class or any combination of the 6 classes, highlighting the complexity and heterogeneity of the AD patient population. (Figure 1) When looking at the samples and the perspective classes they are characterized by, 122 samples were characterized by 2 classes, while only 2 samples belonged to all 6 classes.
Overlapping Mechanisms in Alzheimer’s Disease Perspectives and the Role of Microtubule Stability
Each sample being characterized by more than one perspective class can be explained by the overlapping components across some of the pathways present in each perspective class. For example, 44 samples were classified by both metal ion transport and mitochondrial perspective classes. Of note is that microtubule stability was implicated by the generated hypotheses. In fact, this was part of both the vasculogenesis and metabolism disease paths. This suggests that a potential common mechanism involving microtubules is affecting certain patients and driving disease progression. Specifically, GTSE1 and TUBB1 were both part of this hypothesis in addition to other genes that are being considered as potential therapeutic targets for neurodegeneration.
Sex-based Differences in Perspective Classes of Alzheimer’s Disease Progression
Alzheimer’s has been reported to have greater prevalence and severity in women. We studied if this was also the case in the 6 perspective classes of AD characterization and progression. Although there was a greater number of females than males associated with each perspective class, this is due to a total of 266 female samples and 90 male samples in the dataset. (Figure 2) This apparent sampling bias needs to be taken into consideration when drawing insights in disease etiology.
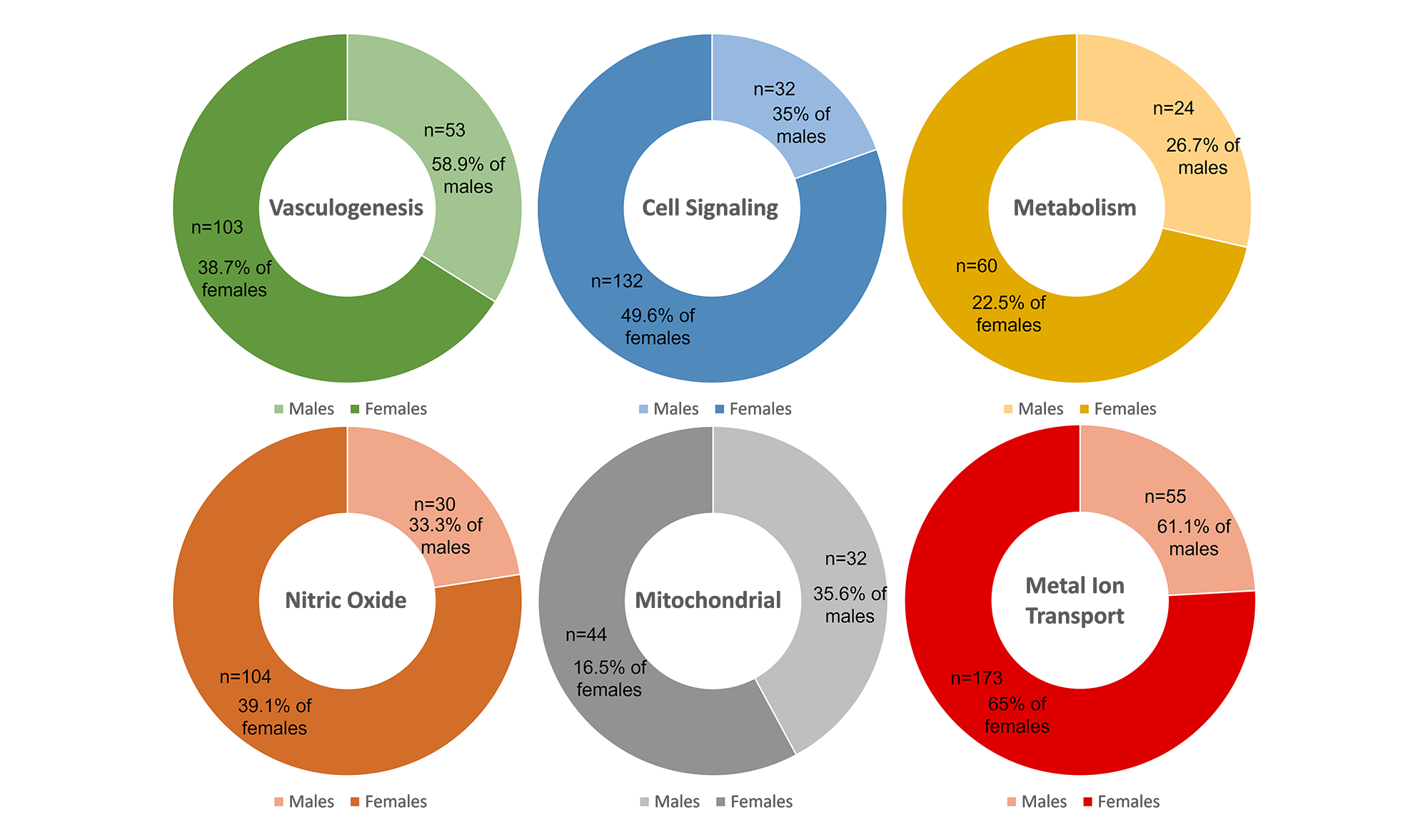
Figure 2. Sex-based differences in the perspective classes of Alzheimer’s Disease.
When looking at the total number of samples in each class, the metal ion transport class was found to be the most common, with 228 samples, 65% of the female and 61% of the male samples progressing via this class. Taking into account the percentage of male and female samples in each class, among the 266 female AD samples, 49.6% progressed through cell signaling compared to only 35.5% of the male samples. Conversely, 58.9% of male AD samples were in the vasculogenesis perspective class compared to 38.7% of female samples. This suggests that gender differences for AD may be more complex than currently understood, as these differences may depend on etiological subtypes.
Significance of Findings
AD is widely known as a heterogeneous disease, yet often AD drug trials have broad inclusion criteria and don’t account for disease heterogeneity in the respective trial design. NetraAI uses a perspective analytics approach that is able to help stratify patients to lead the way toward personalized medicine in AD drug development. The 6 perspective classes identified highlight various mechanisms that contribute to AD heterogeneity and have been implicated in the disease. In addition to the individual classes, looking at the overlap in their underlying mechanisms highlights an additional layer of complexity, particularly with the role of microtubules in the vasculogenesis and metabolism perspective classes, which can explain the efficacy of taxanes and microtubule-targeting drugs in restoring lost nerve signals in AD.
Using artificial intelligence (AI), we have the potential to create a new taxonomy of disease and improve the understanding of complex disorders. Even with a smaller sample size, NetraAI is capable of uncovering multiple genetically driven subtypes and can open up new avenues for improving clinical trial success rates through a finer granularity of understanding. This heightened granularity can help with the creation of new study designs that are optimized based on newly uncovered subtypes.
References
-
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017 – 2025.; 2017. Accessed February 3, 2023. http://apps.who.int/bookorders.
- Tsay Mi, Geraci J, Agrawal A. Next-Gen AI for Disease Definition, Patient Stratification, and Placebo Effect. doi:10.31219/OSF.IO/PC7AK
- GEO Accession viewer. Accessed February 15, 2023. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE84422
- Qorri B, Tsay M, Agrawal A, Au R, Geraci J. Using machine intelligence to uncover Alzheimers disease progression heterogeneity. Explor Med. 2020;1(6):377-395. doi:10.37349/EMED.2020.00026

